Question
Medium
Solving time: 3 mins
(i) Write the electron-dot structures for sodium, oxygen and magnesium. (ii) Show the formation of and by the transfer of electrons. (iii) What are the ions present in these compounds?
Found 5 tutors discussing this question
Discuss this question LIVE
5 mins ago
 Text solution
Text solution Verified
Verified
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
(i) The electron-dot structures for sodium and oxygen
 (ii) Formation of and by the transfer of electrons
Formation of Magnesium oxide:
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing two electrons, the magnesium atoms form a magnesium ion and by gaining two electrons, the oxygen atom forms an oxide ion .
Formation of Sodium oxide:
Two sodium atoms transfer their 2 outermost electrons to an oxygen atom. By losing two electrons, the two sodium atoms form sodium ions . And by gaining two electrons, the oxygen atom forms an oxide ion .
(ii) Formation of and by the transfer of electrons
Formation of Magnesium oxide:
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing two electrons, the magnesium atoms form a magnesium ion and by gaining two electrons, the oxygen atom forms an oxide ion .
Formation of Sodium oxide:
Two sodium atoms transfer their 2 outermost electrons to an oxygen atom. By losing two electrons, the two sodium atoms form sodium ions . And by gaining two electrons, the oxygen atom forms an oxide ion .
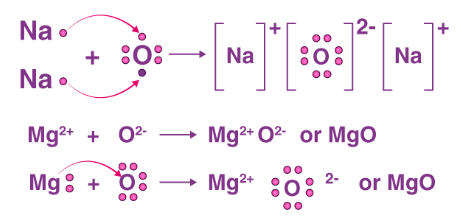 (iii) The ions present in sodium oxide compound are sodium ions ( and oxide ions .
The ions present in the Magnesium oxide compound are magnesium ions and oxide ions .
(iii) The ions present in sodium oxide compound are sodium ions ( and oxide ions .
The ions present in the Magnesium oxide compound are magnesium ions and oxide ions .
Was this solution helpful?
57
Share
Report
Filo tutor solutions (13)
Learn from their 1-to-1 discussion with Filo tutors.
4 mins
Uploaded on: 11/20/2022
Was this solution helpful?
124
Share
Report
2 mins
Uploaded on: 9/27/2023
Was this solution helpful?
63
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice more questions from Science (NCERT)
Q1
Which of the following pairs will give displacement reactions?
Q2
(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of and by the transfer of electrons.
(iii) What are the ions present in these compounds?
Q3
Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
View allPractice questions from Science (NCERT)
Question 2
Easy
Views: 5,984
Practice more questions from Metals and Non-metals
Question 1
Easy
Views: 5,783
The atomic numbers of four elements P, Q, R and S are 6, 10, 12 and 17 respectively. Which two elements can combine to form a covalent compound?
Practice questions on similar concepts asked by Filo students
Question 1
Views: 5,429
Question 2
Views: 5,509
Question 4
Views: 5,970


Stuck on the question or explanation?
Connect with our Science tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of and by the transfer of electrons.
(iii) What are the ions present in these compounds? |
| Updated On | Sep 27, 2023 |
| Topic | Metals and Non-metals |
| Subject | Science |
| Class | Class 10 |
| Answer Type | Text solution:1 Video solution: 13 |
| Upvotes | 1479 |
| Avg. Video Duration | 7 min |





