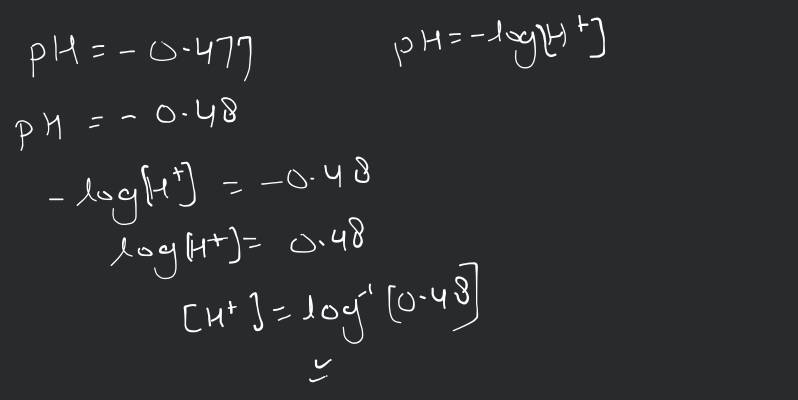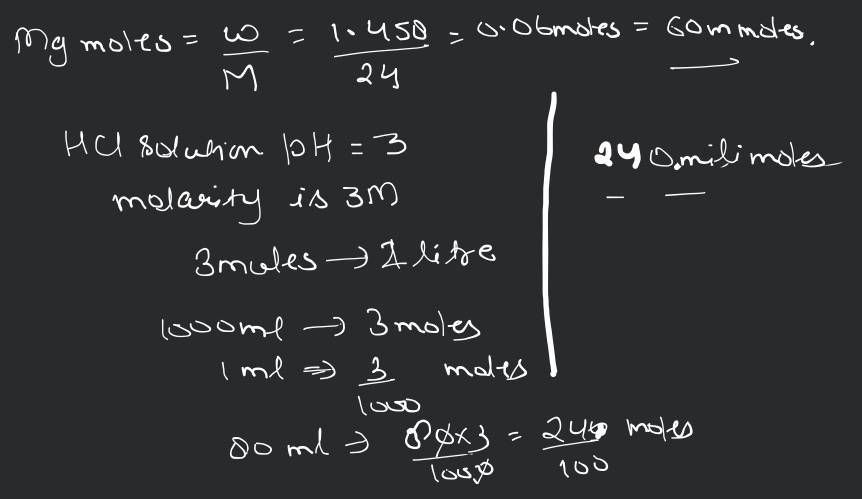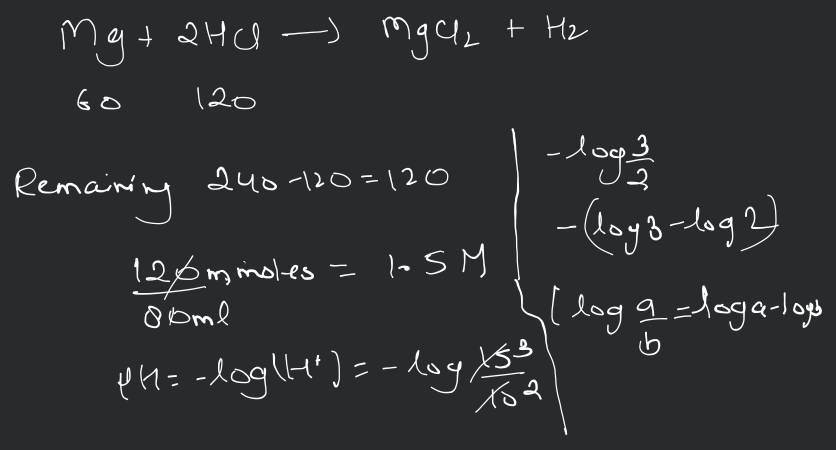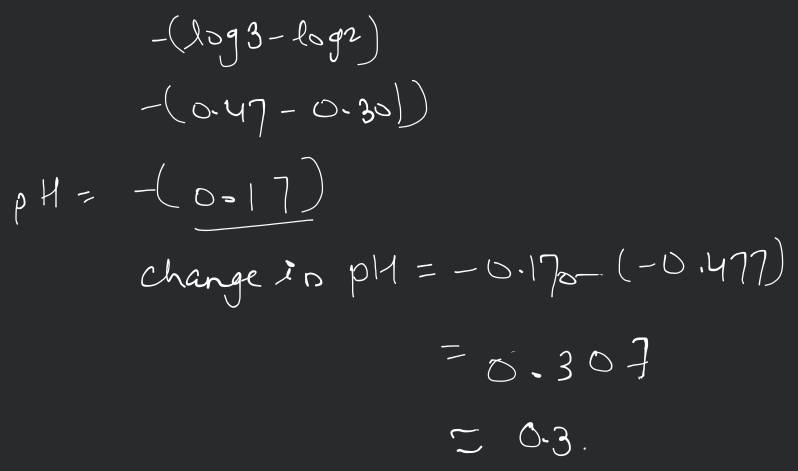Question
Medium
Solving time: 5 mins
The ionization constant of benzoic acid is and for silver benzoate is . How many times is silver benzoate more soluble in a buffer of compared to its solubility in pure water?
Found 6 tutors discussing this question
Discuss this question LIVE
12 mins ago
 Text solution
Text solution Verified
Verified
Let M be the solubility of silver benzoate in water.
. This is solubility of silver benzoate in pure water.
Benzoate ions combine with protons to form benzoic acid but hydrogen ion concentration is constant due to buffer solution.
Let y M be the soubiity in the buffer solution.
Thus, silver benzoate is 3.32 times more soluble in buffer.
. This is solubility of silver benzoate in pure water.
Benzoate ions combine with protons to form benzoic acid but hydrogen ion concentration is constant due to buffer solution.
Let y M be the soubiity in the buffer solution.
Thus, silver benzoate is 3.32 times more soluble in buffer.
Was this solution helpful?
89
Share
Report
Filo tutor solutions (7)
Learn from their 1-to-1 discussion with Filo tutors.
14 mins
Uploaded on: 4/10/2023
Was this solution helpful?
56
Share
Report
20 mins
Uploaded on: 6/12/2023
Was this solution helpful?
64
Share
Report

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice more questions from Chemistry Part-I (NCERT)
Q1
Explain why pure liquids and solids can be ignored while writing the equilibrium constant expression?
Q2
The ionization constant of benzoic acid is and for silver benzoate is . How many times is silver benzoate more soluble in a buffer of compared to its solubility in pure water?
Q3
Reaction between nitrogen and oxygen takes place as follows:
If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
View allIf a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
Practice questions from Chemistry Part-I (NCERT)
Question 1
Medium
Views: 6,231
Question 2
Medium
Views: 5,866
Question 3
Medium
Views: 5,287
is at . If initially and bar and pure graphite is present, calculate the equilibrium partial pressures of and .
Question 4
Hard
Views: 6,232
If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
Practice more questions from Equilibrium
Question 2
Hard
Views: 5,192
Practice questions on similar concepts asked by Filo students
Question 2
Views: 5,348
Question 3
Views: 5,228
Question 4
Views: 5,141


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | The ionization constant of benzoic acid is and for silver benzoate is . How many times is silver benzoate more soluble in a buffer of compared to its solubility in pure water? |
| Updated On | Aug 29, 2023 |
| Topic | Equilibrium |
| Subject | Chemistry |
| Class | Class 11 |
| Answer Type | Text solution:1 Video solution: 7 |
| Upvotes | 694 |
| Avg. Video Duration | 11 min |