Question
Hard
Solving time: 6 mins
Reaction between nitrogen and oxygen takes place as follows:
If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
Found 3 tutors discussing this question
Discuss this question LIVE
12 mins ago
 Text solution
Text solution Verified
Verified
Let moles of take part in the reaction. According to the equation, moles of will react to form moles of . The molar concentration per litre of different species before the reaction and at the equilibrium point is:
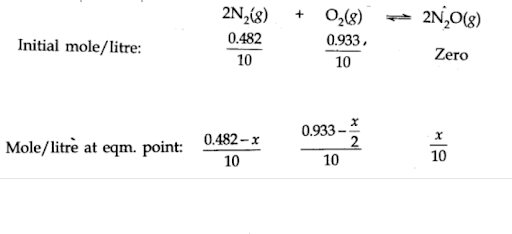
The value of equilibrium constant is extremely small. This means that only small amounts of reactants have reacted. Therefore, is extremely small and can be omitted as far as the reactants are concerned.
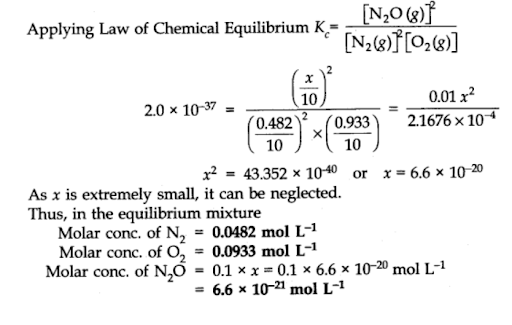
The value of equilibrium constant is extremely small. This means that only small amounts of reactants have reacted. Therefore, is extremely small and can be omitted as far as the reactants are concerned.
Was this solution helpful?
81
Share
Report
Filo tutor solutions (2)
Learn from their 1-to-1 discussion with Filo tutors.

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Practice more questions from Chemistry Part-I (NCERT)
Q1
The ionization constant of benzoic acid is and for silver benzoate is . How many times is silver benzoate more soluble in a buffer of compared to its solubility in pure water?
Q2
Reaction between nitrogen and oxygen takes place as follows:
If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture.
Q3
Nitric oxide reacts with Br2 and gives nitrosyl bromide as per reaction given below: 2NO (g) + Br2 (g) 2NOBr (g) When 0.087 mol of NO and 0.0437 mol of Br2 are mixed in a closed container at constant temperature, 0.0518 mol of NOBr is obtained at equilibrium. Calculate equilibrium amount of NO and Br2 .
View allPractice questions from Chemistry Part-I (NCERT)
Question 1
Medium
Views: 6,275
Question 2
Medium
Views: 6,383
Question 3
Easy
Views: 6,057
Calculate for the equilibrium.
Practice more questions from Equilibrium
Question 1
Hard
Views: 6,087
Question 2
Medium
Views: 5,934
Practice questions on similar concepts asked by Filo students
Question 1
Views: 5,872
Question 2
Views: 5,716
Question 4
Views: 5,492


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | Reaction between nitrogen and oxygen takes place as follows: If a mixture of of and of is placed in a reaction vessel of volume and allowed to form at a temperature for which , determine the composition of the equilibrium mixture. |
| Updated On | Feb 13, 2024 |
| Topic | Equilibrium |
| Subject | Chemistry |
| Class | Class 11 |
| Answer Type | Text solution:1 Video solution: 2 |
| Upvotes | 228 |
| Avg. Video Duration | 9 min |



