Question
Question asked by Filo student
What is the beta decay equations?
Found 6 tutors discussing this question
Discuss this question LIVE
12 mins ago
 Text solution
Text solution Verified
Verified
Beta decay occurs when a neutron located in the nucleus of a radioactive isotope is converted into a proton by the emission of an electron.
In addition to the electron, or -particle, an electron neutrino is also emitted from the nucleus.
Since a neutron is converted into a proton, the atomic number of the element will Increase by 1. At the same time, the mass number will be left unchanged.
You can write the general equation for beta decay like this.
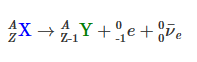
Notice that the atomic number, , increased by , and the mass number, , remained unchanged. You can use that equation to describe the beta decay of any nuclide.
For example, the beta decay of carbon-14 will look like this
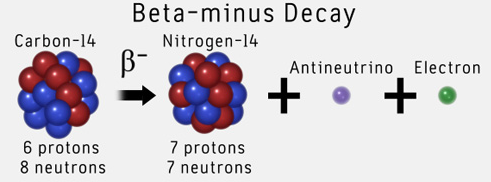
In addition to the electron, or -particle, an electron neutrino is also emitted from the nucleus.
Since a neutron is converted into a proton, the atomic number of the element will Increase by 1. At the same time, the mass number will be left unchanged.
You can write the general equation for beta decay like this.
Notice that the atomic number, , increased by , and the mass number, , remained unchanged. You can use that equation to describe the beta decay of any nuclide.
For example, the beta decay of carbon-14 will look like this

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Students who ask this question also asked
Question 1
Views: 5,305
Question 2
Views: 5,441
Question 3
Views: 5,631


Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | What is the beta decay equations?
|
| Topic | Nuclear Chemistry |
| Subject | Chemistry |
| Class | High School |
| Answer Type | Text solution:1 |



