Question
Question asked by Filo student
How do you graph Boyle's law?
Found 3 tutors discussing this question
Discuss this question LIVE
12 mins ago
 Text solution
Text solution Verified
Verified
Explanation:
So, you would do an experiment in which you measure the volume of a gas at various pressures.
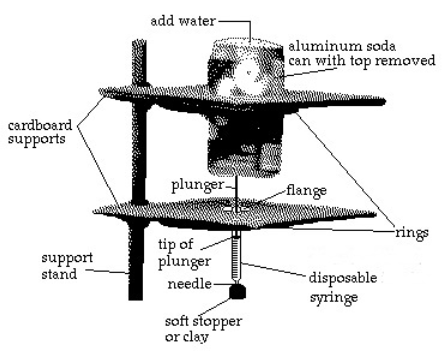
A common lab experiment uses a pop can with various amounts of water to compress the air in a syringe.
Let's assume you get the following data.
The best way to determine a relationship is to plot a graph that gives a straight line.
Since the pressure is directly proportional to the mass of the can plus water, we can use the mass instead of pressure.
At this point we (theoretically) don't know the relationship, so we plot vs. Mass to see what the plot looks like.
We plot pressure as the independent variable (along the horizontal or axis) and volume as the dependent variable (along the vertical or axis).
You could do this by hand, but it is more convenient to use a computer to do the job for you.
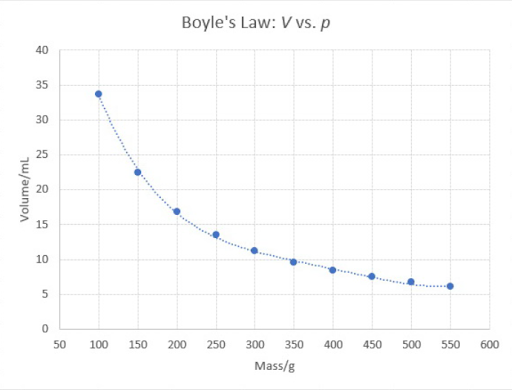
Your graph looks like a hyperbola, as in the diagram above.
The equation for a hyperbola is .
It looks as if we should try a plot of vs. .
We calculate the values of and then plot vs. .
We get the straight line plot shown below.

You can extend the line backwards until it reaches .
If you use a computer or a calculator, you can tell it to calculate the equation for the line that best fits all the points (the regression line).
My computer tells me that the equation is
The graph of against is a straight line through the origin.
This means that the measured volume is inversely proportional to the pressure Boyle's Law.
So, you would do an experiment in which you measure the volume of a gas at various pressures.
A common lab experiment uses a pop can with various amounts of water to compress the air in a syringe.
Let's assume you get the following data.
The best way to determine a relationship is to plot a graph that gives a straight line.
Since the pressure is directly proportional to the mass of the can plus water, we can use the mass instead of pressure.
At this point we (theoretically) don't know the relationship, so we plot vs. Mass to see what the plot looks like.
We plot pressure as the independent variable (along the horizontal or axis) and volume as the dependent variable (along the vertical or axis).
You could do this by hand, but it is more convenient to use a computer to do the job for you.
Your graph looks like a hyperbola, as in the diagram above.
The equation for a hyperbola is .
It looks as if we should try a plot of vs. .
We calculate the values of and then plot vs. .
We get the straight line plot shown below.
You can extend the line backwards until it reaches .
If you use a computer or a calculator, you can tell it to calculate the equation for the line that best fits all the points (the regression line).
My computer tells me that the equation is
The graph of against is a straight line through the origin.
This means that the measured volume is inversely proportional to the pressure Boyle's Law.

One destination to cover all your homework and assignment needs
Learn Practice Revision Succeed

Instant 1:1 help, 24x7
60, 000+ Expert tutors

Textbook solutions
Big idea maths, McGraw-Hill Education etc

Essay review
Get expert feedback on your essay

Schedule classes
High dosage tutoring from Dedicated 3 experts
Students who ask this question also asked
View more

Stuck on the question or explanation?
Connect with our Chemistry tutors online and get step by step solution of this question.
231 students are taking LIVE classes
| Question Text | How do you graph Boyle's law?
|
| Topic | Gases |
| Subject | Chemistry |
| Class | High School |
| Answer Type | Text solution:1 |



